Copper-mediated synthesis of quinazolin-4(3H)-ones from N-(quinolin-8-yl)benzamide and amidine hydrochlorides†
Abstract
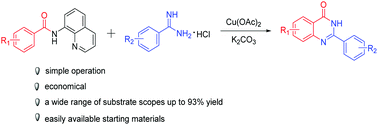
An efficient copper-mediated tandem C(sp2)–H amination to provide quinazolinones from N-(quinolin-8-yl)benzamide and amidine hydrochlorides has been developed. It can afford rather complex products in a single step synthesis from easily available starting materials using 8-aminoquinoline as a removable bidentate directing group. The features of this reaction are it being simple to operate and avoiding sensitive and expensive metals, and it provides an approach for the construction of polycyclic molecules in the area of organic chemistry.


 Please wait while we load your content...
Please wait while we load your content...