New palladium(ii) complexes with 3-(2-pyridyl)-5-alkyl-1,2,4-triazole ligands as recyclable C–C coupling catalysts†
Abstract
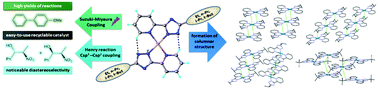
A series of palladium(II) complexes with 3-(2-pyridyl)-5R-1,2,4-triazoles Pd(LR)2 (R = ethyl (4a), n-propyl (4b), i-propyl (4c) and t-butyl (4d)) have been synthesised and characterised by NMR, IR, UV-vis spectroscopy, ESI and MALDI-TOF mass spectrometry. Single-crystal X-ray diffraction study of 4a–d revealed a square-planar coordination geometry, in which the N^N chelating monoanionic ligands adopt a trans-configuration around Pd(II) and show notable intraligand C–H⋯N hydrogen bonding within the complex. The solid state emission and excitation spectra of 4a–d showed vibronic structure of the spectra at room temperature. The TG/DTA curves of 4a–c revealed that the complexes are stable up to ca. 250 °C, whereas 4d starts to decompose at 230 °C. Compounds 4a–d efficiently catalyse the nitroaldol (Henry) reaction of benzaldehyde with nitroethane in water, methanol and ethanol with appreciable diastereoselectivity in favour of the syn-isomer (syn : anti up to 81 : 19). In addition, 4a–d catalyse the microwave-assisted Suzuki–Miyaura cross-coupling reaction of bromoanisole with phenyl boronic acid, in the presence of a base. Moreover, these heterogeneous catalysts can be recovered and reused without losing activity for several consecutive cycles.


 Please wait while we load your content...
Please wait while we load your content...