An efficient clean methodology for the C–S coupling to aryl thioethers and S–S homocoupling to aromatic disulfides catalyzed over a Ce(iv)-leucine complex immobilized on mesoporous MCM-41
Abstract
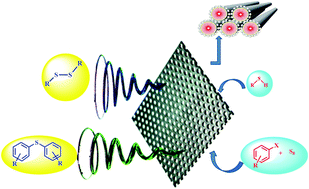
A novel Ce(IV)-anchored L-leucine covalently bonded to mesoporous MCM-41 has been synthesized by a non-hydrothermal post-functionalization approach. It has been thoroughly characterized by sophisticated physicochemical techniques. The material was applied in the efficient green synthesis of aromatic sulfides by C–S coupling using molecular sulfur and haloarenes. Another catalytic application was in the synthesis of symmetric disulfides by the homocoupling of aromatic thiols in the presence of H2O2 as an oxidant. The ligand-free protocol is simple, clean and free from hazardous chemicals. Moreover, the catalyst is reusable for several times, thus making the methodology sustainably viable.


 Please wait while we load your content...
Please wait while we load your content...