Structural diversity of the complexes of monovalent metal d10 ions with macrocyclic aggregates of iso-tellurazole N-oxides†
Abstract
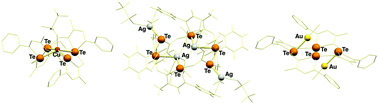
The reactions of iso-tellurazole N-oxides with monocations of the coinage metals yielded a family of coordination complexes with a remarkable variety of compositions and structures. All new species were identified by single-crystal X-ray diffraction. In all cases, the macrocyclic aggregation of the organo-chalcogen heterocycles through Te⋯O chalcogen bonding interactions is preserved and the metal ions are coordinated by tellurium. Copper and gold formed complexes with the tetramers. The lighter element acquired a κ4Te,κ1O pentacoordinated square pyramidal structure with a triflate anion in the apical position. The preference of the heavier metal for a linear κ1Te,κ1Cl coordination geometry permitted the formation of a binuclear complex. Silver demonstrated a unique preference for coordinating to the hexamer; two distinct examples showed a structure with a pair of endocyclic κ2Te,κ2O metal ions bridged by two triflate anions; the other two chalcogen atoms are located at trans-annular positions and each coordinates a κ1Te,κ1O,κ1C silver ion, also bonded to an aromatic carbon and another triflate anion.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...