Novel acrylamide/acrylonitrile-tethered carbazoles: synthesis, structural, biological, and density functional theory studies†
Abstract
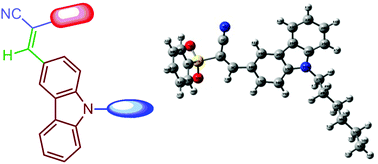
A series of novel carbazolylacrylamides/acrylonitriles 6a–6h were synthesized and their structures were established using various analytical, spectroscopic, and single-crystal X-ray diffraction techniques. The antioxidant evaluation of the target chemical entities 6a–6h was conducted by the DPPH method. The carbazolylacrylonitrile 6h displayed 65.7% radical scavenging activity (IC50 value, 65.08 μM) with respect to standard ascorbic acid. The in vitro cytotoxic activity studies revealed 6e and 6h as promising anticancer molecules against a human pancreatic cancer cell line, i.e., AsPC1. Density functional theory studies of a model chemical entity 6g were performed and the results obtained were compared with those obtained experimentally. The appropriate structure, the corresponding bonding features and the vibrational frequencies for the molecule 6g were determined by employing the DFT-B3LYP method with the 6-311++G(d,p) basis set. The vibrational frequencies of the carbazolylacrylonitrile 6g calculated theoretically were found to be in good agreement with the corresponding experimental results. In addition, the chemical shifts of 1H and 13C of 6g were computed by the gauge independent atomic orbital (GIAO) method and compared with the experimental ones.


 Please wait while we load your content...
Please wait while we load your content...