Oxovanadium(v)-catalyzed deoxygenative homocoupling reaction of alcohols†
Abstract
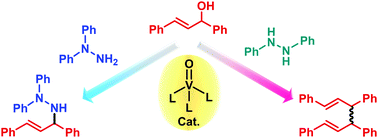
Oxovanadium(V)-catalyzed transformation of alcohols in the presence of hydrazine derivatives was demonstrated. The direct hydrazination reaction of 1,3-diphenylprop-2-en-1-ol with 1,1-diphenylhydrazine in the presence of VO(OSiPh3)3 as a catalyst and MS3A as a dehydrating reagent proceeded to afford the corresponding hydrazination product. On the contrary, the utilization of 1,1-dimethylhydrazine instead of 1,1-diphenylhydrazine was found to induce the deoxygenative homocoupling reaction of the allyl alcohol to give the corresponding 1,5-diene as a major product. In addition to the deoxygenative homocoupling product, the allyl amine into which aniline was introduced was also obtained by using 1,2-diphenylhydrazine in the reaction of 1,3-diphenyl-2-methylprop-2-en-1-ol. Oxovanadium(V)-catalyzed deoxygenative homocoupling reaction of benzyl alcohols could also be performed in the presence of 1,1-dimethylhydrazine.
- This article is part of the themed collection: Vanadium Science: Chemistry, Catalysis, Materials, Biological and Medicinal Studies


 Please wait while we load your content...
Please wait while we load your content...