Polymer-incarcerated palladium-catalyzed facile in situ carbonylation for the synthesis of aryl aldehydes and diaryl ketones using CO surrogates under ambient conditions†
Abstract
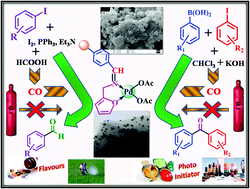
In this existing work, an efficient polymer-supported palladium catalyst, a furfurylamine-functionalized Merrifield complex of palladium [Pd@(Merf-FA)], was synthesized and characterized, showing excellent catalytic activity towards in situ carbonylation reactions using carbon monoxide surrogates like formic acid and chloroform. Herein, we examined the catalytic activity of the Pd@(Merf-FA) catalyst for the formylation of aryl iodides and carbonylative Suzuki–Miyaura coupling reactions. The Pd@(Merf-FA) catalyst was systematically characterized by several techniques like HRTEM, elemental mapping, PXRD, TGA-DTA, FESEM, UV-vis, EDAX, CHN and AAS analysis. The catalyst is highly recyclable, able to be recycled up to six times without showing any significant decrease in catalytic activity. The [Pd@(Merf-FA)] catalyst proved to be more efficient compared to the corresponding homogeneous palladium catalyst. In addition, the leaching experiment of the synthesized catalyst was studied, which showed that negligible leaching of metal occurred from the polymeric support.


 Please wait while we load your content...
Please wait while we load your content...