Redox-switching of ternary Ni(ii) and Cu(ii) complexes: synthesis, experimental and theoretical studies along with second-order nonlinear optical properties†‡
Abstract
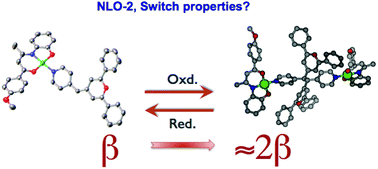
Four new ternary Ni(II) and Cu(II) complexes of ONO tridentate Schiff base and pyridylmethylenepyran (PyMP) ligands [(R-ONO)M(PyMP)] (R = anisyl = An: M = Ni 1, Cu 2; R = ferrocenyl = Fc: M = Ni 3, Cu 4) have been synthesized under facile reaction conditions starting from R-ONOH2, metal(II) nitrate salts, and PyMP; R-ONOH2 stands for the Schiff base ligand precursors obtained by condensation of either 1-anisyl- or 1-ferrocenyl-butan-1,3-dione and 2-aminophenol. They have been thoroughly characterized with the help of various physicochemical tools, such as CHN analyses, IR and UV-vis spectra, 1H NMR for diamagnetic Ni(II) derivatives 1 and 3, and HRMS for paramagnetic Cu(II) species 2 and 4. The molecular structures of 1–3 were authenticated by single-crystal X-ray diffraction methods, along with that of the doubly phenoxide-bridged dimer [Cu2(μ-ONO-Fc)2(PyMp)2] (4′), resulting from the recrystallization of 4. In 1–3, the four-coordinate nickel and copper atoms adopt a square planar geometry, whereas in 4′ the CuII metal ion is five-coordinated in a square pyramidal environment with the pyridyl nitrogen occupying the apex. Electrochemical studies reveal two well-separated redox waves. The spin density distribution analyses reveal that the initial oxidation process is associated with a ligand-based level, with some ferrocenyl participation for the heterobimetallic compounds 3 and 4. Reversible redox switching can be established for 1 and 3 by time-resolved spectroelectrochemistry under thin-layer conditions where electrochemical cycling is associated with a significant modification of the UV-vis spectra of the chromophores. The second-order nonlinear optical responses of 1–4 along with those of assumed bispyrylium dimeric species 5 and 6, generated by chemical oxidation of 1 and 3, respectively, have been determined by harmonic light scattering measurements in dichloromethane solutions at 1.91 μm incident wavelength. Rather high β values ranging from 270–530 × 10−30 esu were determined for 1–4. The β value of 5 (800 × 10−30 esu) was found to be almost twice that of its monomeric precursor 1, whereas the β value of 6 (160 × 10−30 esu) is reduced by half with respect to that of 3. In 5 the anisyl retains its donor ability whereas in 6 the electron-donating character of the oxidized ferrocenyl moiety is cancelled. Optimized geometries of the four compounds 1–4 as well as their electronic structures and that of their respective cations 1+–4+ have been analyzed through DFT calculations, while TD-DFT computation has been used to interpret the major features of the UV-vis spectra.


 Please wait while we load your content...
Please wait while we load your content...