Synthesis of pyramidal tetraarylborate pentads†
Abstract
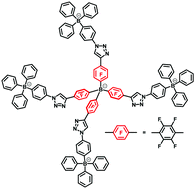
In the present study, we designed tetrahedral tetraarylborate pentads which were synthesized by a typical click reaction, copper-catalyzed azide–alkyne cyclization. The synthesis of the borate pentads was confirmed by FT-IR and NMR spectroscopies, and NMR measurements indicated a rapid exchange of bound and unbound counter cations. The obtained borate pentads exhibited a representative behavior of weak electrolytes, and thus a decrease of their concentration caused a rapid increase of their molar conductivity, especially at the limit of dilution. Additionally, the observed association constant did not correspond to the theoretical association constant, probably because of the multivalent ionic dissociation dependent on the dielectric constant of the media.


 Please wait while we load your content...
Please wait while we load your content...