Mechanistic insight into the silver-catalyzed cycloaddition synthesis of 1,4-disubstituted-1,2,3-triazoles: the key role of silver†
Abstract
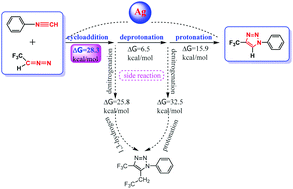
The silver-catalyzed cycloaddition synthesis of 1,4-disubstituted-1,2,3-triazoles was investigated by using density functional theory (DFT) calculations. In this article, we report the computational studies on the main reaction mechanism, two proposed side reaction mechanisms and the role of silver(I) in elucidating the principal features of the transformation. The mechanism exploration indicates that the [3+2]-cycloaddition is more likely to be the rate-determining step for the main reaction, whereas both competitive side reaction pathways have relatively large barriers and are kinetically unfavorable. Frontier Molecular Orbital (FMO) analysis implies that the coordination of silver(I) with isocyanides changes the characteristics of the isocyanide FMO and the manner of FMO overlap in the cycloaddition. Natural Population Analysis (NPA) charge analysis suggests that the electron-withdrawing property of silver(I) leads to the decrease in the charge density of the –NC group, which improves the reactivity and substituent tolerance of isocyanides. Furthermore, the effect of substituents on the aryl rings on –NC groups is almost negligible as compared with the strong electron-withdrawing effect of silver(I) on isocyanides. Hence, silver(I) as the catalyst is thought to be responsible for the excellent aryl-substituted tolerance as well as high regioselectivity of the protocol.


 Please wait while we load your content...
Please wait while we load your content...