Structures, relative stability and binding energies of neutral water clusters, (H2O)2–30†
Abstract
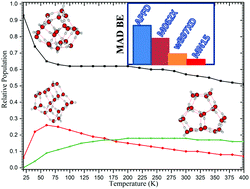
We have revised the structures of neutral water clusters, (H2O)n=2–30, with the affordable M06-2X functional, presenting up to 25 isomers for each cluster size. Besides, relative energies, relative free energies and binding energies of all the investigated clusters are also provided. Temperature dependent isomer distributions of the clusters are also presented. As a result, significant new local minima structures are located on the PESs of water clusters. When available our global minima structures are in agreement with the CCSD(T)/CBS reports. We noted that the cage isomer with a central solvated water molecule starts to be the global minimum structure from n = 17. In addition, we found that from n = 19, the cage isomers are the global minimum structure or they are among the most stable structures, on the PESs of water clusters. From the water 26-mer till 30-mer (excluding the 29-mer), the most stable structures are cage isomers with two central solvated water molecules. Moreover, these cage isomers dominate the population of clusters for temperatures ranging from 20 to 400 K. As far as the energetic study is concerned, binding energies of neutral water clusters are computed using M06-2X, APFD, MN15 and ωB97XD functionals associated with the aug-cc-pVTZ (aVTZ) basis set for n = 2–30. As a result, the MN15/aVTZ binding energies are reported to be in good agreement with the CCSD(T)/CBS benchmark results for n = 2–10. The mean absolute deviation (MAD) for n = 2–10 is calculated to be 1.0 kcal mol−1.


 Please wait while we load your content...
Please wait while we load your content...