A NIR fluorescent sensor for biothiols based on a dicyanoisophorone derivative with a large Stokes shift and high quantum yield†
Abstract
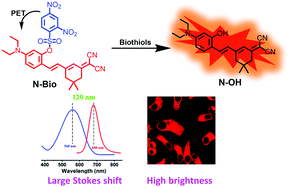
Herein, a NIR fluorescent probe N-Bio was developed for the effective detection of biothiols by exploiting a dicyanoisophorone derivative (N-OH) as the fluorophore and a thiol-responsive 2,4-dinitro-benzenesulfonyl (DNBS) group as the recognition group. The introduction of the N-OH fluorophore endowed the probe with a high quantum yield (0.30), large Stokes shift (120 nm), excellent photostability and high NIR fluorescence inside cells. Upon the cleavage of the DNBS group by biothiols, probe N-Bio demonstrated rapid response (6 min for Cys, 12 min for GSH and 14 min for Hcy), high signal to noise ratios (208-fold for Cys, 204-fold for GSH and 168-fold for Hcy) and exceedingly low detection limits (36.93 nM for Cys, 32.56 nM for GSH, and 65.03 nM for Hcy). Encouraged by the appreciable sensing performance in vitro, N-Bio was then successfully applied for real-time monitoring of the level of biothiols in living cells and real sample detection.


 Please wait while we load your content...
Please wait while we load your content...