Arsenic sensor development based on modification with (E)-N′-(2-nitrobenzylidine)-benzenesulfonohydrazide: a real sample analysis†
Abstract
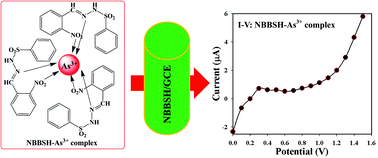
(E)-N′-(2-Nitrobenzylidene)-benzenesulfonohydrazide (NBBSH) was prepared from 2-nitrobenzaldehyde and benzenesulfonylhydrazine by using a simple condensation process with medium yield. It was then crystallized in methanol and characterized using various spectroscopic techniques such as Fourier transform infra-red spectroscopy (FTIR), ultra-violet visible spectroscopy (UV-vis), proton nuclear magnetic resonance (1H-NMR), X-ray photoelectron spectroscopy (XPS), and carbon-13 nuclear magnetic resonance (13C-NMR). The structure of the NBBSH molecule was confirmed using the single crystal X-ray diffraction technique (SCXRDT). A thin layer of NBBSH slurry was deposited onto a cleaned and dried flat round surface of GCE with a binding agent (Nafion) to fabricate a sensitive and selective heavy metal ion (HMI) sensor. The fabricated NBBSH/GCE sensor exhibited enhanced sensing performances such as sensitivity, limit of detection (LOD), linear dynamic range (LDR), and long-term stability towards selective arsenic ions. The calibration curve (CC) was found to be linear over a broad range of As3+ conc. (0.1 nM–0.1 M) and the calculated sensitivity and LOD (based on 3N/S) were found to be ∼190.0 pA μM−1 cm−2 and 50.0 pM, respectively. This novel approach can be used as an efficient path for the development of HMI sensors regarding monitoring of hazardous materials in biological and environmental sciences.


 Please wait while we load your content...
Please wait while we load your content...