Interface engineering in the BNNS@Ti3C2 intercalation structure for enhanced electrocatalytic hydrogen evolution†
Abstract
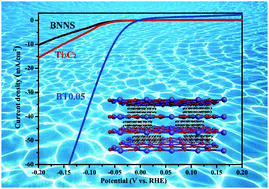
Electrocatalysts with the advantages of stability, high efficiency, and noble-metal-free features are in urgent need for water splitting. Herein, for the first time, based on the interface engineering, a novel BNNS@Ti3C2 intercalation electrocatalyst was prepared via a controllable synthesis strategy. The rich active sites of Ti3C2 were better protected as well as were able to serve as a bridge to connect the different layers of BNNS. Furthermore, combining the first-principles calculations, the nature of the interface proved the transformation from semiconducting properties to metallicity in this unique intercalation structure. The as-obtained composite possessed improved conductivity and abundant catalytic active sites, exhibiting a low onset potential of 23 mV and overpotential of 52 mV (vs. RHE) at 10 mA cm−2 with outstanding stability. BNNS@Ti3C2 was used as an electrocatalyst for the first time without noble-metal assistance. This study demonstrates that the layered materials can serve as a promising electrocatalyst by interfacing with the intercalation structure.


 Please wait while we load your content...
Please wait while we load your content...