Transition-metal-free S–N bond formation: synthesis of 5-amino-1,2,4-thiadiazoles from isothiocyanates and amidines†
Abstract
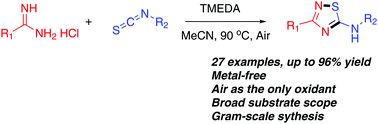
A novel and green method for the synthesis of 5-amino-1,2,4-thiadiazoles has been developed by the reaction of isothiocyanates with amidines. This protocol which is free of metal, catalyst and iodine involves O2 oxidative S–N bond formation for the synthesis of various 5-amino-1,2,4-thiadiazole derivatives with excellent to good yields. High regioselectivity, mild reaction conditions, broad substrate scope and good functional group tolerance are the highlights of the report.


 Please wait while we load your content...
Please wait while we load your content...