Syntheses and photoluminescence of copper(i) halide complexes containing dimethylthiophene bidentate phosphine ligands†
Abstract
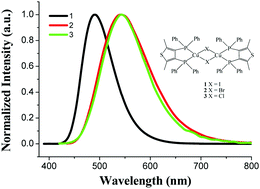
High efficiency copper(I) halide complexes containing rigid diphosphine ligands have attracted much attention. However, rigid bidentate phosphine ligands have been rarely reported so far. Here, an electron-rich heteroaryl ring grafted with two electron donating methyl groups was first included to construct a rigid bidentate phosphine ligand, three four-coordinate dinuclear copper(I) halide complexes, [CuX(dpmt)]2 (dpmt = 3,4-bis(diphenylphosphino)-2,5-dimethylthiophene, X = I (1), Br (2) and Cl (3)), were synthesized, and their molecular structures and photophysical properties were investigated. The structural analysis reveals that two copper(I) centers are bridged by two halogen ligands to form a dinuclear structure with a four-membered Cu2X2 ring. These complexes exhibit intense bluish green to yellow green emission in the solid state at room temperature and have peak emission wavelengths at 490–543 nm with microsecond lifetimes (τave = 3.9–13.6 μs, Φ = 0.26–0.35). The emission of complex 1 mainly originates from MLCT and ligand-centered transitions. Similar emissive behaviors for complexes 2 and 3 are possibly due to ligand-centered transition. The solution-processed devices of complex 3 exhibit stable yellow emission with CIE(x, y) of (0.42, 0.52) for doped and nondoped devices.


 Please wait while we load your content...
Please wait while we load your content...