Organocatalysis by a multidentate halogen-bond donor: an alternative to hydrogen-bond based catalysis†
Abstract
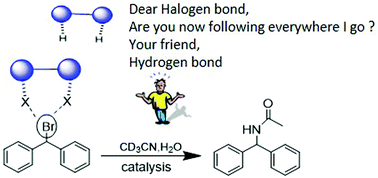
A charge neutral iodoethynyl-based multidentate halogen-bond donor was synthesized and successfully utilized as an organocatalyst in a benchmark Ritter-type solvolysis reaction. The catalytic activity was monitored using 1H NMR spectroscopy and several control experiments were systematically carried out in order to rule out hidden catalysis based on other functional groups.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...
