Low molecular weight supramolecular dehydroepiandrosterone-based gelators: synthesis and molecular modeling study†
Abstract
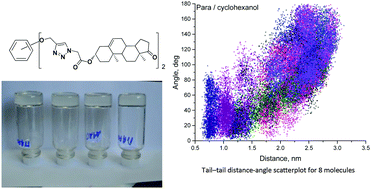
Three novel isomeric supramolecular dehydroepiandrosterone appended 1,2,3-triazole-based potential gelators were synthesized. Their ability to form gels in different solvents was studied experimentally as well as at the molecular level by means of molecular dynamics and quantum chemistry. The conformational changes and binding sites of the molecules were indicated and discussed. While the gelator molecule unfolding may be one of the prerequisites for gel formation, no straight correlation was found. We suggest two ways of gelation of the compounds under study. These ways result from the competition of the molecular entanglement and binding site interactions, depending strongly on the molecular spacer structure.


 Please wait while we load your content...
Please wait while we load your content...