Colorimetric sensing of Fe3+ and Hg2+ and photocatalytic activity of green synthesized silver nanoparticles from the leaf extract of Sonchus arvensis L.†
Abstract
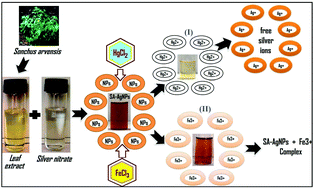
In the present study, an eco-friendly method was reported for the green synthesis of silver nanoparticles (AgNPs) using Sonchus arvensis (SA) leaf extract. Phytochemical analysis of plant extract revealed the presence of flavonoids, tannins, glycosides, and alkaloids, which might be used as a potential capping, reducing, and stabilizing agent in the process. SA-AgNPs were found to be stable with no evidence of agglomeration after one week. UV-vis spectroscopy showed a salient SPR peak of Ag0 at 440 nm, and the calculated bandgap (3.2 eV) explained the semiconducting behaviour of the NPs. The FT-IR spectra elucidated the functional groups of SA extract attached with Ag0. TEM and XRD revealed the spherical shape of SA-AgNPs with an average particle and crystalline size of 25 ± 5 and 16 ± 1 nm, respectively. A colorimetric method was employed for the quick detection of heavy metals in aqueous medium using SA-AgNPs. The obtained NPs were found to be highly selective and sensitive to Fe3+ and Hg2+ metal ions at a detection limit of 10−3 M and thereby act as noble solid bio-sensors. SA-AgNPs showed strong catalytic efficiency against methylene blue compared to other dyes in the presence of sunlight and completely degraded the dye within 1 hour. This catalytic efficiency was observed to be due to the lower bandgap value (3.2 eV) of SA-AgNPs.


 Please wait while we load your content...
Please wait while we load your content...