New insight into the chemistry of selenoureas: synthesis and single crystal structural study of diverse derivatives†
Abstract
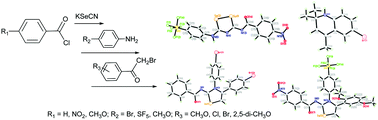
Reacting benzoyl chloride with KSeCN in acetone at room temperature, followed by treatment in situ with 4-bromoaniline led to N-((4-bromophenyl)carbamoselenoyl)benzamide in 93% yield. The same reaction was observed for 4-methoxybenzoyl chloride and 4-pentafluorosulfanylaniline, leading to N-((4-pentafluorosulfanylphenyl)carbamoselenoyl)-4-methoxybenzamide in 76% yield. Interestingly, under the same conditions, 4-methoxybenzoyl chloride and 4-bromoaniline gave rise of the unexpected 2-((4-bromophenyl)amino)-2-methylpropanenitrile and 6-bromo-2,2,4-trimethyl-1,2-dihydroquinoline in 45% and 52% yields. Further, the equivalent reaction of 4-nitrobenzoyl chloride and 4-pentafluorosulfanylaniline afforded an additional 3-(4-nitrobenzoyl)imino-5-(4-(pentafluorosulfanyl)phenylamino)-1,2,4-diselenazole in 19% yield apart from the expected 4-nitro-N-((4-(pentafluorosulfanyl)phenyl)carbamoselenoyl)benzamide in 79% yield. Furthermore, a series of five-membered selenium heterocycles was obtained from the reaction of N-acylselenoureas with various phenacyl bromides. All new compounds were characterised spectroscopically, and twelve X-ray single crystal structures were also discussed.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...