Abstract
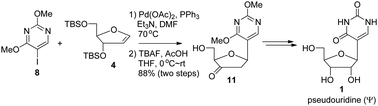
The reaction of 2,4-dimethoxy-5-iodopyrimidine (8) and 3,5-di-O-tert-butyldimethylsilyl protected ribofuranoid glycal 4 was carried out with Pd(OAc)2 as the catalyst, PPh3 as the ligand and Et3N as the base in DMF at 70 °C followed by desilylation to afford exclusively the β-anomer of 5-(2,3-dideoxy-3-oxoribofuranosyl)-2,4-dimethoxypyrimidine (11) in a very good yield. The subsequent protecting group and functional group interconversions furnished pseudouridine (Ψ, 1).


 Please wait while we load your content...
Please wait while we load your content...