Study of the supercapacitive activity of a Eu-MOF as an electrode material
Abstract
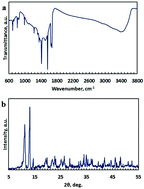
High surface area and redox activity are indispensable characteristics for a material to be considered as an electrode material in pseudocapacitors. In this regard, redox active metal-based metal organic frameworks (MOFs) with high surface area can function as supercapacitors. In this research study, a hydrothermal process was applied to synthesize a Eu-based metal organic framework (Eu-MOF) [Eu2(fma)2(ox)(H2O)4·4H2O] (fma = fumarate and ox = oxalate). The supercapacitive activity of the Eu-MOF was investigated using the instrumental analyses of cyclic voltammetry (CV), galvanostatic charge/discharge, and electrochemical impedance spectroscopy (EIS) measurements in 6 M KOH as an electrolyte. The Eu-MOF exhibited a specific capacitance of 468 F g−1 at a discharge current density of 1 A g−1, and 95.2% capacitance retention after 4000 cycles. To the best of our knowledge, this study is the first to use a lanthanide-MOF as an electrode material for supercapacitors and the results obviously support the outstanding properties of the Eu-MOF for the mentioned application.


 Please wait while we load your content...
Please wait while we load your content...