Positively and negatively charged NHN hydrogen bonds in one molecule: synergistic strengthening effect, superbasicity and acetonitrile capture†
Abstract
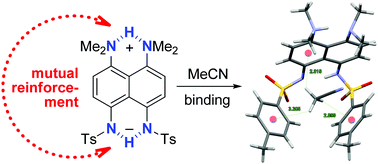
The first zwitterions with [NHN]+ and [NHN]− intramolecular hydrogen bonds have been studied. Due to their mutual reinforcement, shortening of N⋯N distances in both NHN subunits (up to 2.546 Å) occurs in symmetrical zwitterion 12. Being less acidic, the [NHN]− bridge makes the [NHN]+ part harder to disrupt, thus rendering 12 superbasic and recognition properties.


 Please wait while we load your content...
Please wait while we load your content...