Graphitic carbon nitride synthesized by simple pyrolysis: role of precursor in photocatalytic hydrogen production
Abstract
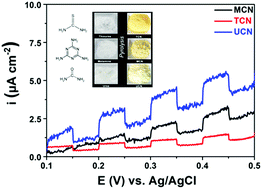
Herein, porous graphitic carbon nitrides (g-C3N4) were synthesized via a one-step pyrolysis process using different low-cost, environmentally benign, nitrogen-rich organic precursors, namely, urea, thiourea, and melamine. The physicochemical and photophysical properties of the obtained g-C3N4 samples were characterized by X-ray diffraction (XRD), solid-state MAS NMR, X-ray photoelectron microscopy (XPS), transmission electron microscopy (TEM), N2 adsorption/desorption, thermogravimetric analysis (TGA), UV-Vis diffuse reflectance absorption spectra (DRS), photoluminescence and electrochemical measurements. This study shows that the photocatalytic hydrogen evolution under visible light is around five times higher if urea is used as the g-C3N4 precursor instead of thiourea or melamine. This finding is confirmed by the lower degree of polymerization of the g-C3N4 sample formed from urea, leading to an altered porous structure, higher pore volume, and enhanced surface area. Moreover, the resulting structural imperfections lead to slightly more active sites as indicated by the most negative conduction band potential in urea-based g-C3N4.


 Please wait while we load your content...
Please wait while we load your content...