Fine-tuning the optoelectronic chattels of fluoreno-thiophene centred molecular semiconductors through symmetric and asymmetric push–pull switch†
Abstract
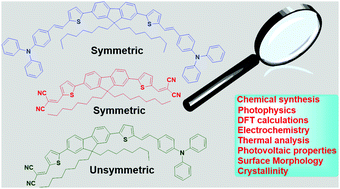
Three new π-conjugated semiconductors of the type D–π–D, A–π–A (symmetric), and D–π–A (asymmetric) were constructed on fluoreno-thiophene spacer by permutation of the lateral groups such as diphenylaminostyryl and dicyanovinyl as electron donor (D) and acceptor (A), respectively. The influence of the asymmetric push–pull effect (as in D–π–A) on the optical as well as the electrochemical properties has been evaluated and compared with the symmetrical D–π–D and A–π–A systems. Theoretical investigation of the molecular properties using DFT/TD-DFT calculations was found to be in good agreement with the experimental results. The chromophore with an asymmetric push–pull architecture emerged as a better candidate for organic solar cell fabrication in terms of its optical band gap, HOMO–LUMO energy level, surface morphology, crystallinity, and charge carrier mobility. The photovoltaic performance of the synthesized chromophores in the bulk heterojunction solar cells revealed a power conversion efficiency of 2.01% for the asymmetric chromophore (D–π–A), which is better than the symmetrical counterparts (D–π–D and A–π–A). Overall, this work demonstrates the impact of symmetric and asymmetric push–pull switch within the fluoreno-thiophene π-molecular backbone on the modulation of the optoelectronic properties.


 Please wait while we load your content...
Please wait while we load your content...