Experimental and computational studies on the synthesis of diastereoselective natural-based Meldrum spiro dibenzofuran derivatives†
Abstract
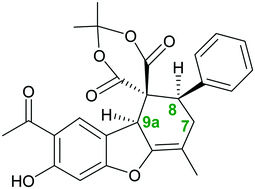
Herein, a novel route to achieve chiral Meldrum spiro dibenzofuran derivatives was developed, which involved a 2,2-dimethyl-1,3-dioxane-4,6-dione (Meldrum's acid)-mediated Knoevenagel reaction of substituted aryl halides, followed by a Diels–Alder reaction with euparin as a natural compound. Various multi-substituted Meldrum spiro dibenzofuran derivatives with very high regio- and diastereoselectivity were obtained in excellent yields at room temperature without requiring column chromatography. The synthesized product was further investigated using density functional theory (DFT) to verify the theoretical–experimental reliability; for this purpose, some parameters, including formation energy, solvent energy, chemical hardness, electronic chemical potential, and electrophilicity, were calculated for these compounds in different solvents using the B3LYP/631G(d,p) level of theory. The results of these computations have provided evidence that using quantum-chemical calculations, it is possible to estimate the stability of each product.


 Please wait while we load your content...
Please wait while we load your content...