Hydrodefluorination of functionalized fluoroaromatics with triethylphosphine: a theoretical and experimental study†
Abstract
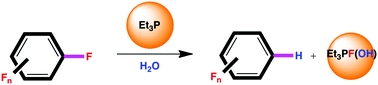
Recently we reported the metal free hydrodefluorination of selected fluoroaromatics using triethylphosphine as the sole defluorinating agent. That prompted us to evaluate the mechanistic proposal and in the light of these results, along with new experimental evidence, we have now modified the initial proposal. The new mechanism avoids the highly energetic β-elimination step of roughly 71 kcal mol−1 for hexafluorobenzene and pentafluoropyridine at 393.15 K, invoking the participation of water. The use of D2O confirmed the role of water as the hydrogen source, yielding the corresponding deutero-defluorinated products; DFT calculations agree with this new proposed mechanism. We also report herein the use of this one-pot hydrodefluorination method applied to a broader number of fluoroaromatic derivatives; some of them allowed the collection of key mechanistic evidence.


 Please wait while we load your content...
Please wait while we load your content...