A new efficient domino approach for the synthesis of coumarin-pyrazolines as antimicrobial agents targeting bacterial d-alanine-d-alanine ligase†
Abstract
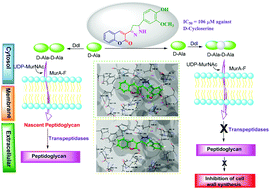
The inhibition of D-alanine-D-alanine ligase (Ddl) prevents bacterial growth, which makes this enzyme an attractive and viable target in the urgent search for novel effective antimicrobial drugs. In this work, a series of novel coumarin-linked pyrazoline inhibitors of D-alanine-D-alanine ligase were synthesized and evaluated as inhibitors of Escherichia coli DdlB ligase in order to target resistant strains of bacteria using environmentally benevolent β-cyclodextrin as a supramolecular catalyst via one-pot four component synthesis in water as a green reaction media. All the newly synthesized compounds have been characterized by elemental analysis and various spectroscopic methods. The new procedure has noteworthy advantages including easy work-up, short reaction times, high yields of products and column-free synthesis. The synthesized compounds were evaluated in vitro for their antimicrobial activity. Among the synthesized compounds, namely 3-(5-(4-hydroxy-3-methoxyphenyl)-4,5-dihydro-1H-pyrazol-3-yl)-2H-chromen-2-one (5f) was found to be the most potent D-alanine-D-alanine ligase enzyme inhibitor, with an IC50 value 106 μM, and the compound 3-(5-(p-tolyl)-4,5-dihydro-1H-pyrazol-3-yl)-2H-chromen-2-one (5g) was found to be the second-most potent inhibitor of the DdlB enzyme, with an IC50 value 111 μM against the standard D-cycloserine. In addition, SAR study provided evidence that the –OH, –CH3 and –OCH3 groups at the 4- and 3-position of the coumarins linked to the pyrazolines scaffold increased enzymatic inhibition, while the molecular docking study of most active compounds 5a, 5g, and 5j against DdlB enzyme of E. coli exhibited good binding properties. This work thus highlights the coumarin-linked pyrazoline motif as a very promising tool for the development of novel antimicrobial compounds acting through an interesting bactericidal mechanism of action.


 Please wait while we load your content...
Please wait while we load your content...