Mild and selective silicon-mediated access to enantioenriched 1,2-mercaptoamines and β-amino arylchalcogenides†
Abstract
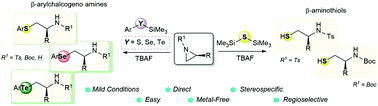
Metal-free ring opening reactions of activated and unactivated aziridines with different silyl chalcogenides are described. Judicious tuning of the reaction conditions enables the synthesis of chiral enantioenriched N-Ts and N-Boc 1,2-mercaptoamines in good yields from the corresponding aziridines and bis(trimethylsilyl)sulfide. N-Protected and N-H unactivated aziridines are efficiently converted into the corresponding β-arylchalcogeno amines upon treatment with suitable arylchalcogenosilanes. The silicon-mediated ring opening reactions proceed with excellent regioselectivity and stereospecificity, allowing to access a wide array of synthetically and biologically valuable enantioenriched chalcogenoamines.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...