Synthesis and characterization of 3-O-esters of N-acetyl-d-glucosamine derivatives as organogelators†
Abstract
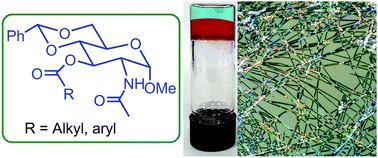
Carbohydrate derived low molecular weight organogelators are interesting compounds with many potential applications. Selective functionalization of the different hydroxyl substituents on D-glucose and D-glucosamine resulted in small molecular gelators. Previously we have found that the C-2 acylated derivatives including esters and carbamates of 4,6-O-benzylidene protected glucose and glucosamine derivatives have shown remarkable applications as molecular gelators. In this research, in order to probe the structural influence of sugar derivatives on molecular self-assembly, we introduced acylation functional groups to the 3-hydroxyl group of 4,6-O-benzylidene acetal protected N-acetyl glucosamine derivatives. A library of fourteen ester derivatives was synthesized and characterized. The ester derivatives typically formed gels in pump oil and aqueous mixtures of dimethyl sulfoxide or ethanol. The resulting gels were characterized using optical microscopy, and rheology, etc. All alkyl ester derivatives were gelators for pump oil. A short chain ester derivative was able to form gels in a few different oils and the corresponding oil water mixtures phase selectively. The compound was also used to trap naproxen sodium and formed a stable co-gel. The controlled release of the drug from the gel to the aqueous phase was analyzed using UV-vis spectroscopy. These results show that the functionalization at the 3-OH position of the N-acetyl glucosamine derivative is a feasible strategy in designing new classes of organogelators.


 Please wait while we load your content...
Please wait while we load your content...