Tuning water oxidation reactivity by employing surfactant directed synthesis of porous Co3O4 nanomaterials†
Abstract
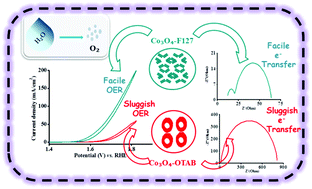
We have explored Co3O4 based nanomaterials for the oxygen evolution reaction prepared via a surfactant directed soft-templating strategy. The surfactants were non-ionic Pluronic® F127 and ionic octadecyl trimethylammonium bromide (OTAB). The study revealed a Brunauer–Emmett–Teller (BET) surface area of 89 m2 g−1 for Co3O4 synthesized from Pluronic® F127 having narrow mesopores in an unrestricted fashion with sheet like morphology. However, Co3O4 synthesized from OTAB provided pores in a restricted manner with low intensity of mesopores, spherical morphology and a BET surface area of 46 m2 g−1. Electrochemical studies revealed that the reactivity of Co3O4 synthesized from F127 towards the oxygen evolution reaction (OER) was much superior, with an outstanding mass activity of 123.1 A g−1 and turnover frequency of 0.286 s−1. Impedance spectroscopy further disclosed that interfacial resistance and resistance towards surface adsorbed intermediates formation during OER decreased significantly, presumably due to the material's narrow mesopores which improves the electrochemically accessible surface area, while the sheet like morphology improved the electrical conductivity.


 Please wait while we load your content...
Please wait while we load your content...