On the importance of antiparallel π–π interactions in the solid state of isatin-based hydrazides†
Abstract
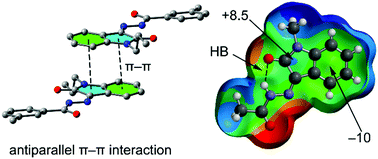
The condensation of N-propyl isatin (1) with different carboxylic acid hydrazides (RCONHNH2 (2–6), R = arene) via sonication was used to synthesize five new hydrazones (7–11). Fully characterized molecular structures were further studied by single-crystal X-ray diffraction showing them to be Z-(syn-) form with respect to the hydrazoic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. In the crystal structures, hydrogen bonds and π-stacking interactions are described and analyzed by means of density functional theory (DFT) calculations since they play a crucial role in the construction of three-dimensional supramolecular frameworks. Moreover, the noncovalent interactions have been characterized using the NCIplot index. Remarkably, the π-system of the substituted isatin ring presents a dual character (acidic/basic), thus promoting the formation of the antiparallel π-stacking assemblies to maximize electron donor–acceptor π–π interactions. Moreover, interesting cooperativity effects have been studied since the presence of an intramolecular H-bonding interaction enhances the strength of the π–π interactions as shown by DFT calculations.
N bond. In the crystal structures, hydrogen bonds and π-stacking interactions are described and analyzed by means of density functional theory (DFT) calculations since they play a crucial role in the construction of three-dimensional supramolecular frameworks. Moreover, the noncovalent interactions have been characterized using the NCIplot index. Remarkably, the π-system of the substituted isatin ring presents a dual character (acidic/basic), thus promoting the formation of the antiparallel π-stacking assemblies to maximize electron donor–acceptor π–π interactions. Moreover, interesting cooperativity effects have been studied since the presence of an intramolecular H-bonding interaction enhances the strength of the π–π interactions as shown by DFT calculations.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...