Turn-on fluorescent sensor for the detection of lipopolysaccharides based on a novel bispyrenyl terephtalaldehyde-bis-guanylhydrazone†
Abstract
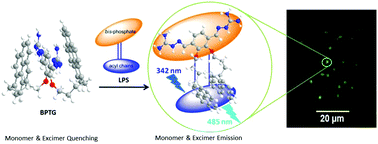
A novel bispyrene compound (BPTG) was developed as a selective lipopolysaccharide (LPS) sensor. The BPTG probe exhibited high selectivity and sensitivity toward LPS with a fluorescence ‘off–on’ behavior in HEPES-buffered DMSO–H2O (1 : 6 (v/v), HEPES = 10 mM, pH = 7.4) with a low detection limit of 5 nM. The turn-on flurescence sensing of the LPS occurred through monomer and excimer emissions. The mechanism of the probe was supported by computational experiments and was found to be unique for its sandwich conformation and self-quenching ability at ground state prior to the binding to the LPS with a butterfly-like skeleton. Upon binding with LPS in an aqueous medium, the probe showed a dose-dependent increase in fluorescent emissions and exhibited a typical excimer emission peak at 485 nm along with a monomer emission peak at 375 nm. BPTG is highly selective for LPS over heparin and other anionic biological species. Due to the expression of LPS on the cell surface of Gram negative bacteria, BPTG was successfully applied as a fluorescent dye to visualize live Vibrio cholerae, which are life-threatening bacteria causing diarrhoeal disease.


 Please wait while we load your content...
Please wait while we load your content...