Multifunctional phosphoramide-(S)-prolinamide derivatives as efficient organocatalysts in asymmetric aldol and Michael reactions†
Abstract
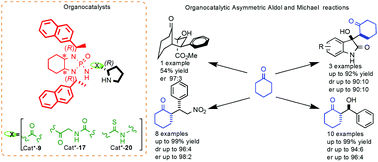
The synthesis and evaluation of three novel chiral organocatalysts derived from (S)-proline and containing a bis-amidophosphoryl amine fragment are reported. The structure and conformation of the new compounds were determined by NMR spectroscopy and X-ray crystallographic analysis. The present study represents an effort directed to enhance the performance of (S)-proline-derived organocatalysts in asymmetric aldol and Michael reactions by means of increased steric interactions arising from the incorporation of naphthyl moieties in the catalysts. In the event, the stereoselectivity achieved with naphthyl substituents turned out to be rather similar to that obtained with phenyl analogs. Nevertheless, the new organocatalysts exhibited rather good enantio- and diastereoselectivities in aldol reactions with various isatins and aryl carbaldehydes, affording products with up to 94 : 6 diastereomeric ratios and enantiomeric ratios as high as 95 : 5. Furthermore, products obtained from Michael addition reactions exhibited up to 96 : 4 diastereomeric ratios and enantiomeric ratios as high as 98 : 2.
- This article is part of the themed collection: Celebrating recent chemical science in Mexico


 Please wait while we load your content...
Please wait while we load your content...