A mechanism of the luminescent covalent organic framework for the detection of NH3†
Abstract
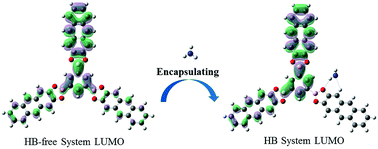
The interaction between a luminescent covalent organic framework (COF) and the indoor pollutant NH3 was investigated based on density functional theory and time-dependent density functional theory. The frontier molecular orbitals and electronic configuration showed that the luminescence mechanism of the COF was strongly affected by the hydrogen bond between the COF and NH3. In addition, the calculated hydrogen bond length, infrared (IR) spectra, and proton nuclear magnetic resonance (1H NMR) spectroscopy analysis indicated that the hydrogen bond in the S1 state was enhanced, which is identical to the calculated results of electronic excitation energies. The fluorescence rate coefficient of the COF was reduced when interacting with NH3. The hydrogen bond between the COF and NH3 in the S1 state is critical for the COF luminescence properties, which means that the COF shows potential application in the detection of NH3.


 Please wait while we load your content...
Please wait while we load your content...