pH-Dependent photocatalytic performance of modified bismuth vanadate by bismuth ferrite†
Abstract
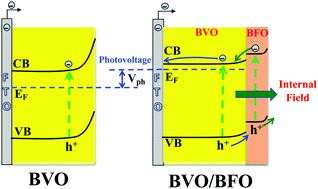
The photoelectrochemical (PEC) performance of bismuth vanadate (BVO) as a promising photocatalyst for water splitting is limited due to the poor charge mobility and significant recombination of electron–hole pairs. Here, we demonstrate that depositing a thin layer of bismuth ferrite (BFO) on the surface of bismuth vanadate (BVO) improved the PEC performance and photocatalytic activity of BVO/BFO over all pH ranges. The BVO/BFO heterojunction exhibited higher photocurrent density and a 0.3 V cathodic shift of the onset potential compared to pure BVO. Moreover, a pH-dependent PEC performance was found for BVO/BFO so that with increase of the pH for BVO/BFO the photocurrent density decreased, while the PEC performance increased for the pure BVO photoelectrode. Interestingly, potential recovery in open current configuration indicated a fast recovery of potential for BVO/BFO compared to BVO, suggesting that the BFO layer in the BVO/BFO heterojunction induces a rapid charge transfer into the electrolyte solution. Electrochemical impedance spectroscopy (EIS) and Mott Schottky measurements indicated that the improved PEC performance of the BVO/BFO heterojunction was attributed to the reduced charge-transfer resistance and the increased donor concentration after BFO deposition on the BVO/BFO heterojunction. Furthermore, we measured the photocatalytic activity of the BVO/BFO catalysts with degradation of tetracycline (TC) under visible light irradiation. The results showed a higher activity for BVO/BFO catalysts compared to BVO or BFO catalysts over the entire pH range.


 Please wait while we load your content...
Please wait while we load your content...