The construction of accelerated catalytic Fenton reaction based on Pd/MIL-101(Cr) and H2
Abstract
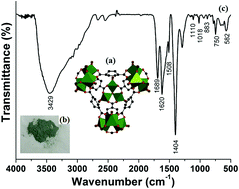
A novel catalytic Fenton system based on H2 and the solid catalyst Pd/MIL-101(Cr) (MHACF-MIL-101(Cr)) was developed at normal temperature and pressure. In this system, the reduction process of FeIII back to FeII was accelerated significantly. Using only trace levels of FeII at the beginning of the reaction, MHACF-MIL-101(Cr) provided continuous production of hydroxyl radicals and rapid degradation of the model contaminant 4-chlorophenol (10 mg L−1) under the initial conditions of pH 3, 25 μM FeII, 25 mM H2O2, 85 mL min−1 H2 and 2 g L−1 Pd/MIL-101(Cr). The activity of the solid catalyst gradually decreased from 100% to about 70% after 6 consecutive degradation reaction cycles of 18 h. This may mainly be attributed to the structural damage and the surface area reduction of this catalyst.


 Please wait while we load your content...
Please wait while we load your content...