An acetylenedicarboxylato-bridged Mn(ii)-based 1D coordination polymer: electrochemical CO2 reduction and magnetic properties†
Abstract
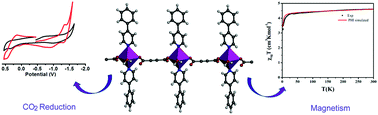
A new Mn(II)-based one-dimensional coordination polymer (1D CP), [Mn(adc)(4-phpy)2(H2O)2]n, (1) (H2adc = acetylenedicarboxylic acid and 4-phpy = 4-phenylpyridine) was synthesized using the linear dicarboxylate adc ligand. The structure of compound 1 was obtained using the single-crystal X-ray diffraction (SCXRD) technique. Inspection of the solid-state structure revealed that compound 1 formed a three-dimensional (3D) supramolecular aggregate using a combination of hydrogen bonding and C–H⋯π interactions. Interestingly, compound 1 was found to behave as an efficient catalyst for the electrochemical reduction of CO2. Variable-temperature magnetic measurements revealed the weak anti-ferromagnetic properties of compound 1.


 Please wait while we load your content...
Please wait while we load your content...