Diastereoselective synthesis of bis(α-aminophosphonates) by lipase catalytic promiscuity†
Abstract
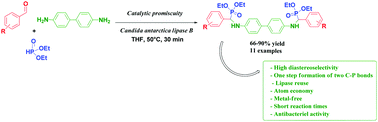
New bis(α-aminophosphonates) were directly prepared with high diastereoselectivity by lipase catalytic promiscuity in the presence of immobilized Candida antarctica lipase. We focused on the multi-component Kabachnik–Fields reaction using various aldehydes, benzidine, and diethylphosphite in one pot. The reaction proceeded with short reaction times with good to excellent yields. The CAL-B was easily recovered and reused several times. A total diastereoselectivity was observed for bis(α-aminophosphonates) 4a, 4c, 4h, 4i, 4k and was high for 4b, 4f and 4j.


 Please wait while we load your content...
Please wait while we load your content...