The first enantioselective strategy towards speciosins†
Abstract
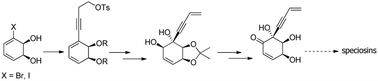
Speciosins constitute a group of natural compounds that have never been synthesized enantioselectively. We propose to introduce chirality by the use of enantiopure 3-halo-cis-1,2-cyclohexadienediols of microbial origin. The introduction of the alkynylic side chain by Sonogashira's methodology was successful, thus confirming a valuable strategy for the asymmetric preparation of speciosins.


 Please wait while we load your content...
Please wait while we load your content...