Properties of iron vanadate over CdS nanorods for efficient photocatalytic hydrogen production
Abstract
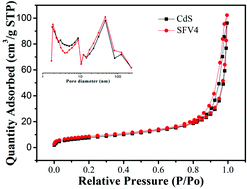
In this study, V3+/VO2+/VO2+ was successfully employed as a redox mediator to modify CdS nanorods for the first time, and remarkable enhancement of efficient hydrogen evolution was obtained. Based on systematic investigations such as UV-vis diffuse reflectance spectra, photoluminescence spectra, transient photocurrent response, XRD, XPS, TEM, SEM, BET and electrochemical impedance spectroscopy to determine the inner reaction mechanism, the dramatically enhanced properties were mainly attributed to the coordinated effects of fast electron transfer. These effects are aided by the various valence states of the vanadate ion centers. Moreover, the effective redox couple strategy of FVO/CdS significantly promotes charge transfer. In addition, these novel composite materials not only exhibit high photoelectrochemical performance, but also effectively prevent photocorrosion of CdS. This work highlights the importance of the redox couple strategy for accelerating charge separation and opens a new avenue for the development of visible light-driven photocatalysts for H2 production.


 Please wait while we load your content...
Please wait while we load your content...