Nickel-catalyzed regioselective C–H halogenation of electron-deficient arenes†
Abstract
A straightforward Ni(II)-catalyzed general strategy was developed for the ortho-halogenation of electron-deficient arenes with easily available halogenating reagents N-halosuccinimides (NXS; X = Br, Cl and I). The transformation was highly regioselective and a wide substrate scope and functional group tolerance were observed. This discovery could be of great significance for the selective halogenation of amides, benzoic esters and other substances with guiding groups. Mechanistic investigations were also described.
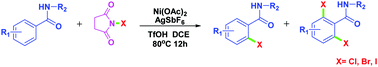


 Please wait while we load your content...
Please wait while we load your content...