Development of new hydantoin-based biocidal polymers with improved rechargeability and anti-microbial activity†
Abstract
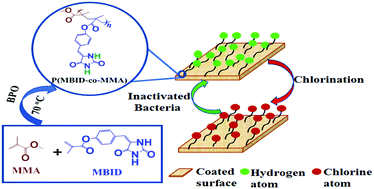
N-Halamines, commonly known as hydantoins, are being increasingly explored for generating antibacterial surfaces. The synthesis of hydantoin-based polymers usually involves the conjugation of polymerizable moieties at the imide position of 5,5′-disubstituted hydantoin, thereby restricting the halogen capture only at the amide nitrogen. In this study, we report the synthesis of a new hydantoin monomer that possesses both amide and imide nitrogens for improved halogen capture and enhanced antibacterial activity. Moreover, unlike previous reports, the synthesized monomer lacks the vicinal C–H group next to N–Cl in the hydantoin ring, preventing HCl elimination upon chlorination and enhancing its antibacterial efficacy, durability and rechargeability. Copolymers of the hydantoin monomer with methyl methacrylate (MMA) and styrene were synthesized and characterized using NMR, HRMS, FTIR, XPS, TGA, DSC and GPC. Polymers containing oxidative chlorine contents varying from 0.85% to 1.35% were employed to modify the glass surface through spin coating. The copolymer coated surfaces exhibited total kill (6 log reduction) of bacteria such as S. aureus and E. coli in 13 min and 15 min, respectively.


 Please wait while we load your content...
Please wait while we load your content...