Synthesis of blue-red emissive amido-substituted di(het)aryl and tri(het)aryl amine derivatives via chemoselective N-mono and N,N-diarylation of (het) aryl amino amides using benzyne/arynes†
Abstract
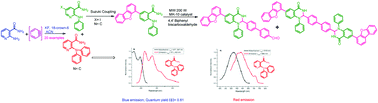
Herein, via chemoselective N-mono and N,N-diarylation of an aryl/hetaryl amino amide reaction using benzyne or arynes, an amide-substituted triaryl amine derivative and diaryl amine derivatives were afforded. The scope and limitation of the present study have been studied. The products thus obtained were synthetically transformed to highly functionalized biphenyl bridged heterocycles via Suzuki coupling and condensation with 4,4′-biphenyl dialdehyde. Evaluation of the photophysical properties has revealed that the triaryl amine derivatives are blue emissive with high quantum yields, whereas the heterocyclic triaryl amine derivatives are blue-red emissive. The benzofuran-derived compound 4i was found to be blue emissive with high quantum yield, whereas the pyridine-derived compound 5j was found to be red emissive with low quantum yield.


 Please wait while we load your content...
Please wait while we load your content...