Organocatalytic enantioselective functionalization of indoles in the carbocyclic ring with cyclic imines†
Abstract
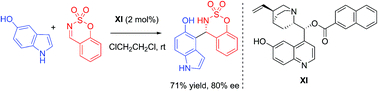
An organocatalytic enantioselective functionalization in the carbocyclic ring of indoles with benzoxathiazine 2,2-dioxides is described using a quinine-derived bifunctional organocatalyst. This aza-Friedel–Crafts reaction provides 4-indolyl, 5-indolyl and 7-indolyl sulfamidate derivatives in good yields (up to 99%) and with moderate to high enantioselectivities (up to 86% ee).


 Please wait while we load your content...
Please wait while we load your content...