T3P® mediated domino C(sp2)–H sulfenylation/annulation of enaminones and methylsulfinyls for the synthesis of chromone thioether derivatives†
Abstract
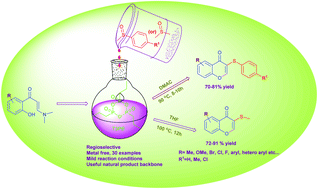
A new regioselective method for the synthesis of 3-(methylthio)-4H-chromen-4-one and 3-(phenylthio)-4H-chromen-4-one derivatives has been developed. The reaction between o-hydroxy-phenyl-functionalized enaminones and methylsulfinyl derivatives using T3P® gave good yields of chromone thioether derivatives. The reaction proceeds via domino chromone ring construction and C(sp2)–H bond sulfenylation under transition-metal-free conditions.


 Please wait while we load your content...
Please wait while we load your content...