Azulenes with aryl substituents bearing pentafluorosulfanyl groups: synthesis, spectroscopic and halochromic properties†
Abstract
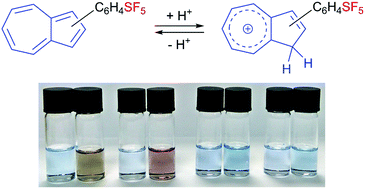
Four regioisomeric azulenes bearing pentafluorosulfanylphenyl substituents have been prepared and characterised by various spectroscopic techniques. The absorption spectra are qualitatively similar in the visible region for all isomers, but upon protonation exhibit pronounced variation dependent on the connectivity within each molecule.
- This article is part of the themed collection: The Mechanics of Supramolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...