Zinc-based CPs for effective detection of Fe3+ and Cr2O72− ions†
Abstract
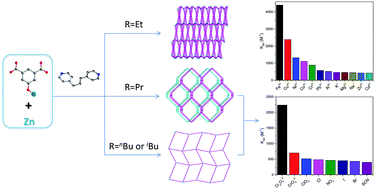
Four new zinc-based coordination polymers (CPs), namely {[Zn(EtOip)(bpp)]·0.5bpp·4H2O}n (1), [Zn(PrOip)(bpp)(H2O)]n (2), {[Zn(nBuOip)(bpp)]·2H2O}n (3), and {[Zn(iBuOip)(bpp)]·2H2O}n (4) (where EtOip = 5-ethoxyisophthalate, PrOip = 5-n-propoxyisophthalate, nBuOip = 5-n-butoxyisophthalate, iBuOip = 5-i-butoxyisophthalate, bpp = 1,3-bis(4-pyridyl)propane), were synthesized from 5-substituted isophthalate ligands using the 1,3-bis(4-pyridyl)propane (bpp) ligand. Topology analysis indicated that compound 1 showed a 3D lvt topology, whereas compound 2 showed a two-fold interpenetrated 3D diamond network. Complexes 3 and 4 exhibited 2D sql/Shubnikov tetragonal plane networks. The coordination polymers were distinguished by the 5-substituted groups of isophthalate and the conformation of bpp. Moreover, compounds 2–4 are bifunctional luminescent materials that can detect environmentally relevant Fe3+ and Cr2O72− ions using a luminescence quenching method. The detection limits of CPs 2–4 for Fe3+ were 6.676 × 10−5 M, 5.442 × 10−5 M, and 1.773 × 10−5 M, respectively, whereas the detection limits of CPs 2–4 for Cr2O72− were 0.1314 × 10−5 M, 1.778 × 10−5 M, and 1.676 × 10−5 M, respectively. The possible mechanisms for the fluorescence quenching phenomenon toward Fe3+ and Cr2O72− ions have also been investigated in detail.


 Please wait while we load your content...
Please wait while we load your content...