Synthesis of spirooxindoles fused with pyrazolo-tetrahydropyridinone and coumarin-dihydropyridine-pyrazole tetracycles by reaction medium dependent isatin-based multicomponent reactions†
Abstract
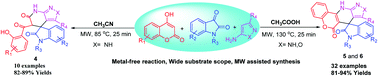
A reaction medium dependent three-component reaction of isatin, 4-hydroxycoumarin and aminopyrazole/aminoisoxazole has been reported under microwave heating conditions for the synthesis of two different types of fused spirooxindoles. The reactions of isatin, aminopyrazole and 4-hydroxycoumarins under microwave irradiation in acetonitrile medium provided spirooxindoles fused with pyrazolo-tetrahydropyridinones by ring opening of the hydroxycoumarin moiety. Similarly, when the same combination was treated in acetic acid as the reaction medium, corresponding fused spirooxindoles having a tetracyclic coumarin-dihydropyridine-pyrazole/isoxazole moiety spiro fused with oxindoles were observed. Using this switchable multicomponent reaction, series of medicinally important spiroxindole fused pyrazolo-tetrahydropyridinones and spirooxindole fused coumarin-dihydropyridine-pyrazole/isooxazole tetracycles have been synthesized under metal-free conditions. The salient features of this methodology are: medium dependent reaction path switching properties, the possibility to generate two types of products from the same starting materials, metal-free reaction conditions, a wide substrate scope, good yields and all the molecules have more than one bioactive moiety.


 Please wait while we load your content...
Please wait while we load your content...