Structure–redox reactivity relationships in Co1−xZnxFe2O4: the role of stoichiometry
Abstract
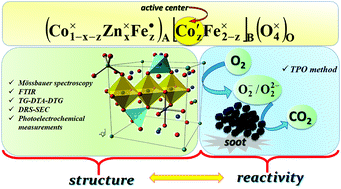
Nanostructured Zn-doped cobalt ferrites (Co1−xZnxFe2O4, where x ranges from 0.0 to 1.0 with a step of 0.1) were studied in order to elucidate the relation between their cationic distribution within the spinel sublattices and catalytic properties. The thermal transformation of the precursors (metal hydroxides) obtained through the hydroxide co-precipitation method was studied by DTA, TGA and FTIR. The thermal behavior of the precursors showed that cobalt ferrite was formed at a lower temperature (376 °C) in comparison to zinc ferrite (478 °C). FTIR analysis revealed vibrational bands around 400 cm−1 and 600 cm−1 related to (MO4)6− and (MO6)9− groups, respectively. The cationic distribution was determined from Mössbauer spectra analysis and the results showed that Zn ions occupy the A-sites, while Co and Fe ions are located in the A- and B-sites. The spectra indicate spinel magnetic ordering in samples with x = 0.0…0.5. The increase in Zn content influences the difference in the Pauling electronegativities, distances between magnetic ions (hopping length) and polaron radius of Co1−xZnxFe2O4 ferrites, inducing changes in the ionic bond strength for the A- and B-sites. Photoelectrochemical measurements demonstrated that the introduction of Zn2+ into the spinel structure modifies the ability of the materials to reduce O2. Whereas photoinduced reduction of dioxygen at CoFe2O4 competes successfully with anodic photocurrent generation (observed as a decrease of photocurrent), for ZnFe2O4 this process is negligible. The relation between the cationic distribution (analyzed in terms of antistructure modeling) and the catalytic activity of spinel ferrites was demonstrated for the example of the soot combustion process. It was shown that the octahedral cobalt centers exhibit a higher catalytic activity than the tetrahedral ones.


 Please wait while we load your content...
Please wait while we load your content...